A new solution for the treatment of diabetic foot ulcers
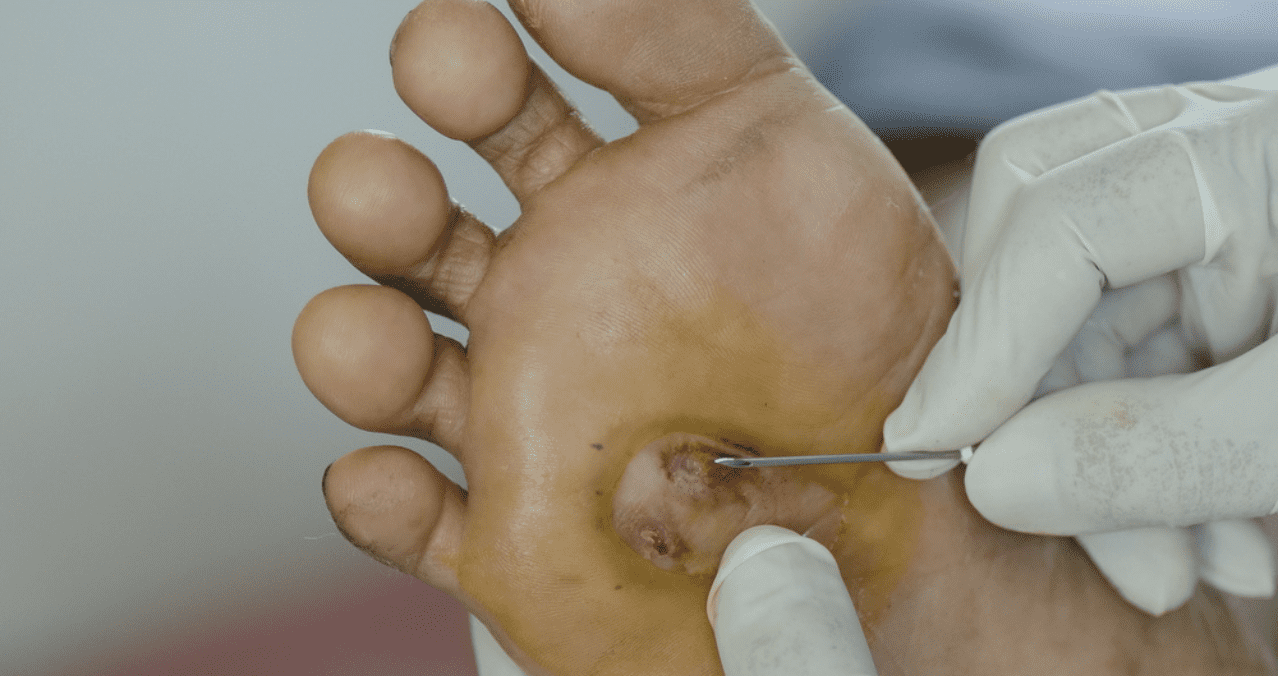
Topical esmolol hydrochloride 14% gel is a new treatment option, which treats diabetic foot ulcers much better than usual treatment and has not caused any major safety issues.
In the study, a significantly higher proportion of patients in the esmolol usual treatment group plus the usual treatment group alone achieved targeted ulcer closure within 12 weeks. Photo: ShutterStock.
A study presented at the 58th annual meeting of the European Association for the Study of diabetic (EASD) 2022, on September 20, evaluated the effectiveness of esmolol hydrochloride gel topical to treat Ulcers Unaffected diabetic foot.
Esmolol hydrochloride is an intravenous (cardio-selective) ß-1-adrenergic receptor blocker that is approved for the treatment of supraventricular tachycardia and rapid ventricular rate heart failure.
Related Studies
Some studies have already demonstrated the anti-inflammatory properties of esmolol and the authors suggested that a topical formulation could stimulate nitric oxide production, which leads to fibroblast migration and endothelial cell mobilization, thus accelerating wound healing in patients with diabetic.
Current research: population and methodology
This study included patients diabetic type 2 which has an area of Ulcers In diabetic foot of 2-15 cm2 and targeted ulcer = 6 weeks. After the week-long screening phase, 176 patients were randomly assigned to receive 14% of esmolol gel. topical At the same time, the usual treatment is for 12 weeks, with follow-up until week 24.
The first thing to do was to follow the recommendations given on the usual treatment by the American Association for diabetic The International Working Group on Diabetic Foot. The primary efficacy endpoint was the proportion of patients who achieved targeted ulcer closure during the 12-week treatment phase. Targeted ulcer closure is defined as 100% re-epithelialization without the need for drainage or dressing.
consequences
A significantly higher proportion of patients in the Esmolol plus usual treatment group achieved targeted ulcer closure within 12 weeks (odds ratio [OR]: 2126; p = 0.0276); Improvements were maintained until the end of the study (week 24; OR: 2.708; p = 0.0126).
In addition, the esmolol plus usual treatment group showed superiority to esmolol alone in each subgroup, regardless of ulcer time, body mass index, hemoglobin concentrations, etc.
From the end of treatment (week 12) to the end of the study (week 24), there was a 60.65% reduction in ulcer area with esmolol + usual care compared to a 2.74% lower decrease with usual treatment alone (p = 0.021), indicating metabolic memory for esmol topical in fibroblast migration.
Esmolol treatment in addition to the usual treatment also reduced secretions significantly faster than the usual treatment alone (p = 0.024). Investigator Dr. Rastogi concluded that none of the reported serious adverse events were associated with esmolol hydrochloride.

“Award-winning zombie scholar. Music practitioner. Food expert. Troublemaker.”


/cloudfront-eu-central-1.images.arcpublishing.com/prisa/AHVYMMDSTZDTDBFNZ3LMFUOKNE.jpg)